
40 Surprising and Interesting Helium Facts
We all have seen balloons at an anniversary party, at work or other particular occasions. Helium is what makes those vibrant balloons float. Here we have compiled a list of 40 interesting Helium Facts in which you can learn about noble gases, the helium atom, chemical properties and much more.
1. The word helium comes from the Greek word helios which means sun.
2. Helium is a chemical element.
3. The symbol of Helium is He.

4. Helium is a tasteless, odorless and colorless gas.
5. Helium was discovered by French astronomer Jules Janssen on August 18th, 1868.
6. The atomic number of Helium is 2.

7. After neon, Helium is the 2nd least reactive noble gas.
8. Helium weighs 0.1785 grams per liter.
9. After Hydrogen, Helium is the second most ordinary element in the globe.

10. The world largest supplier of helium is the USA.
11. Radioactive decay of uranium and thorium produces about 3000 metric tons of helium a year.
12. The boiling point of Helium is the lowest among all the elements.
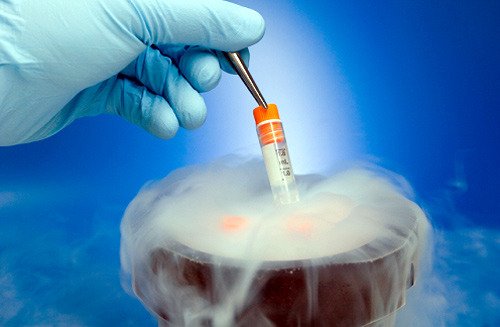
13. Liquid helium is used to cool metals for superconductivity use.
14. Helium became usable in the market for the first time in 1928.
15. Helium is lighter than air, due to this property, it is usually used to fill balloons, airships and blimps.

16. In 1903, large reservoirs of helium were found in U.S. natural gas fields.
17. Liquid helium is used in cryogenics.
18. The Crystal structure of Helium is of Hexagonal shape.

19. The atomic mass of Helium gas is 4.002602 amu.
20. On March March 26, 1895, Scottish chemist, Sir William Ramsay, isolated helium on Earth by treating the mineral cleveite with mineral acids.
21. There are two electrons present in Helium.
22. There are two neutrons and protons present in Helium.
23. The Density of Helium is 0.000164 g/cm3.
24. The melting point of Helium is 0.95K or -457.96 ºF or -272.20 ºC.

25. There are nine isotopes of Helium.
26. Helium-4 makes up about 23% of the universe’s ordinary matter.
27. The atomic radius of Helium gas is 31 pm.
28. Helium can be easily converted into a liquid at an extremely low temperature.
29. In 1881, Italian physicist Luigi Palmieri detected helium on Earth.
30. 3He and 4He are the only stable isotopes of Helium.
31. The isolation process of Helium is called liquefaction.
32. Sir William Ramsey found helium onEarthin 1895.
33. The Boiling Point of Helium is 4.222 K or -452.070 ºF or -2683928 ºC.

34. The outside shell of Helium is filled with electrons. This property of helium makes it unreactive and non-flammable.
35. Helium is continually formed at the inside cores of stars.
36. It is the same gaseous chemical element Helium that’s used in both balloons and MRIs.
37. Helium is the only gas that cannot be made into solid simply by lowering the temperature.
38. Fusion is the process where two hydrogen atoms combine to form a helium atom, releasing energy.
39. Helium is often mixed with oxygen in scuba air tanks to dilute the oxygen.
